There is a relatively new class of medicines collectively called the Biologics which work on the chemical mediators of Psoriasis. Tumor Necrosis Factor alpha (TNF alpha) is an important mediator of Psoriasis which induces the inflammation of Posriasis and there is a class of Biologics called the TNF alpha inhibitors which include Enbrel, Humira and Remicade. These Biologics are indicated for moderate to severe Psoriasis and Psoriatic Arthritis. Enbrel is a fusion protein that blocks TNF alpha and is administered as a subcutaneous injection by the patient twice a week. Remicade is a monoclonal antibody, which is administered by an IV in a physicians office every one to two months. Humira is a monoclonal antibody which is administered as a subcutaneous injection by the patient every two weeks. TNF alpha inhibitors are contraindicated in the following patients: Patients with active infections, being treated for an infection, have or had Hepatitis B infection, take the medicine Kineret (anakinra), have TB or have been in close contact with someone who has TB, have HIV, have multiple sclerosis or Guillain Barre Syndrome, have heart failure or heart conditions, are scheduled for major surgery, are pregnant, plan to become pregnant or breastfeeding, if a patient is allergic to rubber or latex (for Humira and Enbrel as the needle cover of the syringe/pen contains dry natural rubber. The side effects of TNF alpha inhibitors include but are not limited to the following: Immunosuppression- these medicines work by suppressing the immune system so there is an increased risk of infections and cancers including leukemia and lymphoma in patients taking these medicines. The risk of skin cancer is also increased. Re-activation of a previous infection or cancer is also a risk. Vaccines- Do not receive live vaccines while on these medicines. Lupus like Syndrome-This can develop while taking the Biologics. Skin side effects- injection site reactions, rash, skin cancer can occur. Joints- Joint pain may occur. Hematologic- Cell blood counts should be obtained routinely to ensure no problems with the blood lines occur. Caveat- These medicines have undergone extensive clinical trials and have been approved by the FDA; however, they have been on the market a relatively short time so side effects may exist that are not currently known.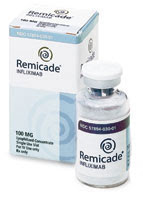
April 3, 2011